~Matter~
Matter is anything that has weight and takes up space. Everything you can see and touch is made up of matter. Matter exists in three main forms: solids, liquids, and gases. It also has properties that we can describe through density, solubility, conductivity, magnetism, etc.
When scientists use the word “matter” they are talking about solids, liquids, and gases. Matter can be found on Earth in three main forms: solids, liquids, and gases. Solids are materials that have a definite shape and volume that stays the same. Rocks are a good example of a solid – they have a rigid shape that isn’t easily changed.
Liquids are a type of matter that changes shape depending on the shape of its container. For example, when you pour milk into a cup, it takes up the cup’s inner shape. Matter that spreads out to take up all the space available in the container is called a gas. Air is a gas. So is helium, which is put inside birthday party balloons.
What’s the Matter?
Today we will be revising the three states of matter and learning more about atoms.We are learning to describe the three states of matter and how to describe the changes of matter and the water cycle.
The three states of matter are:
In a solid the particles are held together too strongly to allow movement from place to place but the particles do vibrate about their position in the structure.
In a liquid the particles are quite close together and move with random motion throughout the container.
In a gas the particles move rapidly in all directions, frequently colliding with each other and the side of the container.
When a solid is heated it melts and becomes a liquid. When a liquid is heated it steam and becomes a gas. When a gas cools it liquefies and becomes a liquid.
Solids, liquids and gases are called the three states of matter. Materials can be changed from one state to another by heating or cooling. Water can be observed as a liquid, a solid (ice), or a gas (water vapour) and moves around the environment in a process known as the water cycle.
Heating
If ice (solid) is heated, it changes to water (liquid). This change is called melting.
- Water (liquid) can change to water vapour (gas). This is called evaporation.
- If water (liquid) is heated until it boils, it changes to water vapour (gas) very quickly. Water boils at 100°C
Cooling
- If water vapour (gas) is cooled, it changes to water (liquid). This change is called condensing.
- If water (liquid) is cooled, it changes to ice (solid). This change is called freezing. Water freezes at 0° C
Solids and liquids
Heat melts a solid and turns it into a liquid. Cooling freezes a liquid into a solid. Different solids melt at different temperatures, some high, some low. These are called their melting points.
Heating
- Heat can change solids into liquids or gases.
- Most solids melt into liquid when they are heated.
- A liquid evaporates into a gas when it is heated.
Cooling
When we cool something we take heat away from it. Cooling changes a gas into a
liquid, and a liquid into a solid.
- A gas condenses into a liquid when it is cooled.
A liquid freezes into a solid when it is cooled.
Melting points
Different solids melt at different temperatures. Ice melts at 0 degrees Celsius (0°C). Chocolate melts at about 35°C. We say that chocolate has a higher melting point than ice. Metals, like aluminium and iron, also melt when we heat them. They have very high melting points. They have to be very hot to melt.
Temperature
Temperature is a measure of how hot or cold things are. You need a thermometer to measure temperature. Temperature is measured in degrees Celsius (°C).
- Ice melts at exactly 0°C.
- A hot bath is about 40°C
- Water boils at exactly 100°C
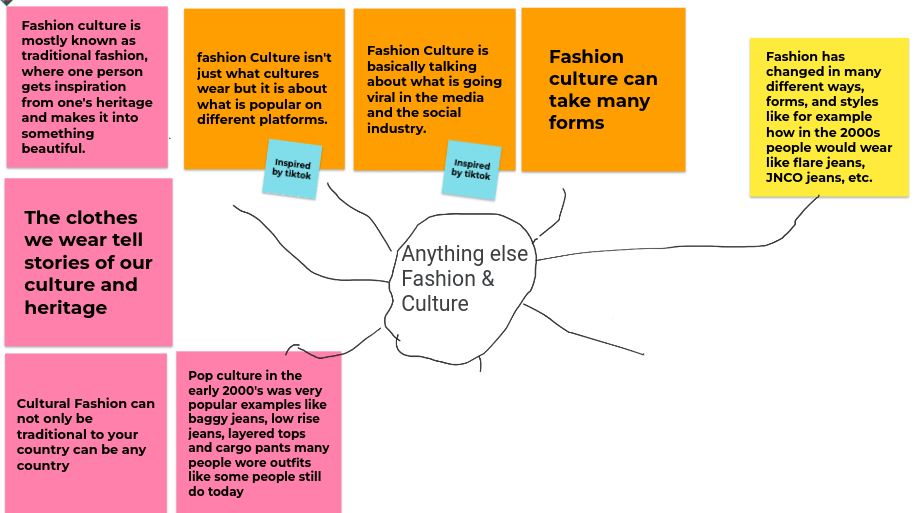
The water cycle
Water on the earth is constantly moving. It is recycled over and over again. This recycling process is called the water cycle.
Water evaporates into the air – The sun heats up water on land, and in rivers, lakes and seas and turns it into water vapour. The water vapour rises into the air.
Water vapour condenses into clouds – Water vapour in the air cools down and changes back into tiny drops of liquid water, forming clouds.
Water falls as rain – The clouds get heavy and water falls back to the earth in the form of rain
or snow.
Water returns to the sea – Rain water runs over the land and collects in lakes or rivers, which take it
back to the sea. The cycle starts all over again.
The world’s water moves between lakes, rivers, oceans, the atmosphere and the land in an ongoing cycle called the water cycle. As it goes through this continuous system, it can be a liquid (water), a gas (vapour) or a solid (ice).
Energy from the sun heats up the surface of the Earth, causing the temperature of the water in our rivers, lakes and oceans to rise. When this happens, some of the water “evaporates” into the air, turning into a gas called “vapour“. Plants and trees also lose water to the atmosphere through their leaves. This process is known as “transpiration“.
As water vapour rises up high into the sky, it cools and turns back into a liquid, forming clouds. This process is called “condensation“. Currents high up in the air move these clouds around the globe.
When too much water has condensed, the water droplets in the clouds become too big and heavy for the air to hold them. And so they fall back down to Earth as rain, snow, hail or sleet, a process known as “precipitation“.
The fallen precipitation is then “collected” in bodies of water, such as rivers, lakes and oceans – from where it will eventually evaporate back into the air, beginning the cycle all over again. How it is collected, depends on where it lands…
~Atoms~
Atoms are the smallest building blocks of matter and make up everything around us. Every atom has a centre called a nucleus, which is made of particles called protons and neutrons. Electrons move in electron shells around the nucleus. Atoms can bond to one another to form solids, liquids, or gases.
What is an atom? Atoms are the smallest building blocks of matter and make up everything around us.
What is a proton? A proton is a tiny particle that is found in the nucleus of an atom.
What is a neutron? Neutrons are the particles in an atom that have a neutral charge.
What is an electron? Electrons are particles charged up with tons of energy.
Each element has a different number of protons. This is how we tell them apart. They also have different numbers of neutrons and electrons, but these can change. Helium has 2 protons so its atomic number is 2, it also has 1 or 2 neutrons and 2 electrons. This is different to Lithium which has 3 protons and an atomic number of 3. It also has 3 electrons and 3 or 4 neutrons. Each element has its own symbol.


~Atoms & Molecules~
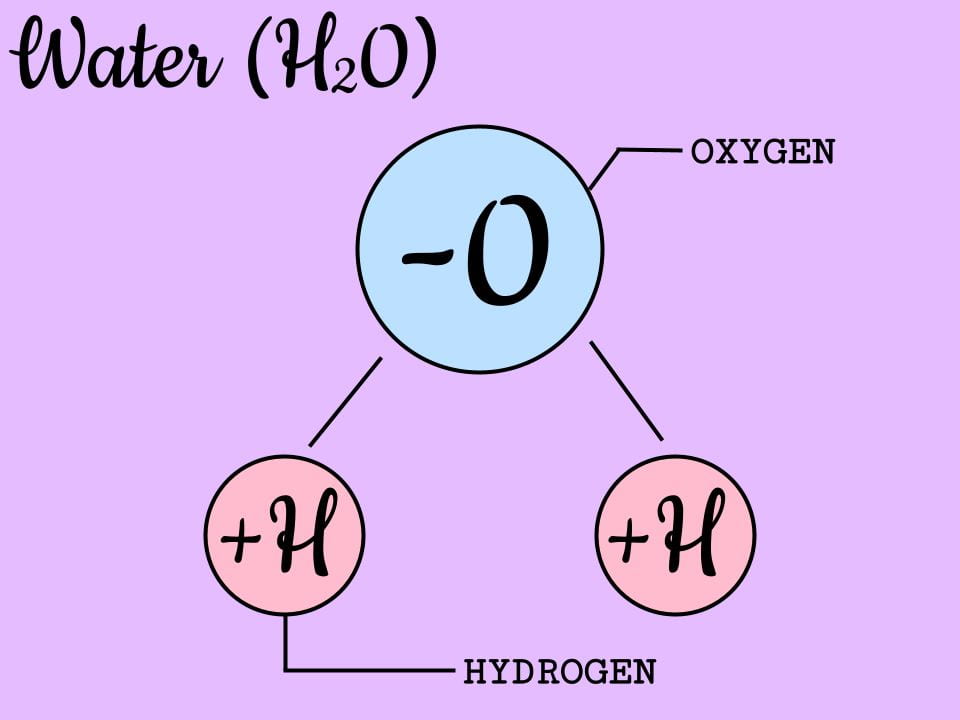
We are learning that everything is made out of different combinations of atoms and that different materials have different properties. Everything is made up of different combinations of these atoms. For example, water molecules are made up of two hydrogen atoms and one oxygen atom. That is why water is also called H2O.
What is the difference between a molecule and an atom?
A molecule is made up of atoms bonded together. So, while an atom is its own separate entity, a molecule is what you get when those atoms bond together. These might be the same elements, such as two oxygen atoms bonded together (O2), or it might be different atoms bonded together like water (H2O).
Atoms also come in different sizes, depending on how many electrons they have. An oxygen atom is a lot (16 x) larger than a hydrogen atom. Draw a picture (and take a photo) or create an image on google draw of a water molecule H20. It contains 2 hydrogen atoms and one oxygen atom.
Different materials have different properties. For example, they have different melting and boiling points, different mass (weight), density and some are stronger than others. This is caused by their molecular structure.
We need to keep this in mind when designing different products. For example, we make saucepans out of metal because they won’t melt on our stoves. However, if we tried to place a plastic pan on the stove it would melt because it has a lower melting point. We can create strong metal by creating a metal alloy (by combining one type of metal with another metal or element). These alloys can be stronger than the metal on its own.
Overview:
Solids, liquids, and gases – The objects around us can take on many forms. They can be solid like a computer screen, which holds together when pushed or prodded. They can be liquids like the water we drink, which flows and changes its shape. Or they can be gases like the invisible air we breathe, which floats around freely. Solids, liquids, and gases are all forms of matter, the stuff that makes up everything around us.
States of matter – Materials in the world around us come in three different forms, which we call states of matter. Objects that hold their shape, like bricks, coins, or blocks of wood, are called solids. Liquids, such as water and oil, are substances that flow, and form a puddle if they are not kept in a container. Gases, like air, are substances that drift around and will float away if they are not sealed up. The arrangement of atoms within a substance determines whether it is a solid, liquid, or gas.
Changing states – We can change a solid into a liquid or gas by changing its temperature. This is known as changing its state. Water is a liquid at room temperature, but becomes a solid (called ice) if it is cooled down. The same water turns into a gas (called water vapour) if it is heated up. The changes only happen when the substance reaches a particular temperature. Water turns to ice at 32°F (0°C). This is known as its freezing point. Water turns to water vapour at 212°F (100°C). This is known as its boiling point. Boiling points and freezing points come at different temperatures for different substances.
What is matter? Matter is another word for the stuff things are made of. Everything around us is made of matter, from the air we breathe to the water we drink-even our own bodies. Planet Earth is made of matter, and so are all the stars, planets, and moons in the universe. All matter is made up of tiny particles called atoms. Matter takes on different forms depending on how the atoms are arranged. We call these forms “states of matter”. On Earth, the most common states are solids, liquids, and gases.
Atoms – All matter is made up of tiny particles called atoms. Atoms are so small that there are 60 million trillion atoms in a single grain of sand (that’s 60,000,000,000,000,000,000). There are 118 kinds of atoms, which make up everything around us, from the smallest piece of dust to entire stars and planets. Each kind of atom is called an element.
Molecules – Atoms are rarely found by themselves— they usually join up with other atoms in groups called molecules. A molecule is two or more atoms joined (or “bonded”) tightly together. The number and kinds of atoms in a molecule, and the way they are arranged, determine what substance it makes. For example, a molecule made of two oxygen atoms joined to one carbon atom forms carbon dioxide, a colourless gas. But a molecule made of two hydrogen atoms joined to one oxygen atom forms water, a liquid.
Inside an atom – Atoms may be tiny, but they are made up of even smaller particles, called subatomic particles. These come in three types: protons, electrons, and neutrons. Protons and neutrons sit together at the centre of the atom, while electrons buzz around them. The number of protons in the nucleus of an atom determines what substance it makes. Atoms with 79 protons inside make gold, a colourful metal. Atoms with eight protons in the nucleus make oxygen, an invisible gas.
~Chemical VS Physical Changes~